Editor's note:
Combining the advantages of carbon and silicon materials while mitigating their disadvantages, these two elements are often combined to maximize their overall utility. Silicon-carbon composite materials can generally be categorized into two types based on the type of carbon used: conventional silicon-carbon composites and new silicon-carbon composites.
Lithium-ion batteries are widely used in portable electronic devices such as computers, mobile phones, and electric vehicles due to their high energy density, high open-circuit voltage, and long cycle life. Currently, lithium batteries are highly commercialized. As one of the four key components of a lithium battery (cathode material, anode material, separator, and electrolyte), the performance of the anode material has a significant impact on the overall battery performance. Graphite is currently the most commonly used anode material, and it belongs to the category of carbon-based anodes, including both natural and artificial graphite.
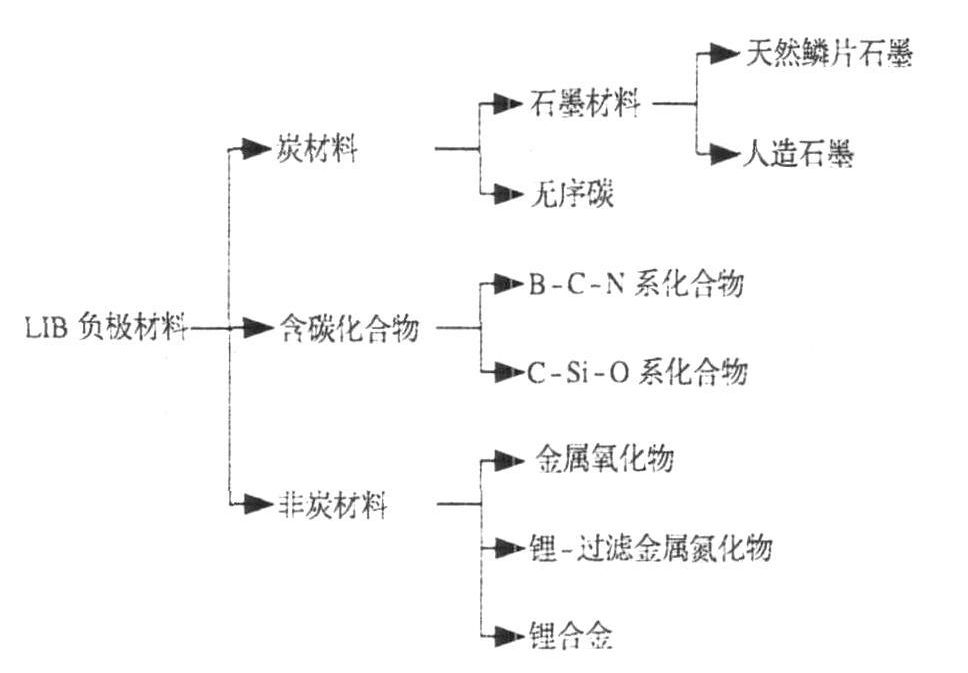
Figure 1. Types of lithium battery anode materials
Graphite is considered an ideal anode material due to its excellent cycle stability, good electrical conductivity, and layered structure that allows for efficient lithium intercalation. However, with increasing performance demands for lithium batteries, the limitations of graphite as a negative electrode have become more apparent. For example, its low specific capacity (around 372 mAh/g), tendency for layer peeling after multiple cycles, and limited potential for improving energy density have driven researchers to seek alternatives.
Silicon has attracted considerable attention as a promising anode material because it can form a binary alloy with lithium and has a much higher theoretical capacity (up to 4200 mAh/g). Additionally, silicon exhibits a low deintercalation voltage (below 0.5 V vs Li/Li+), low reactivity with electrolytes, abundant natural resources, and low cost.
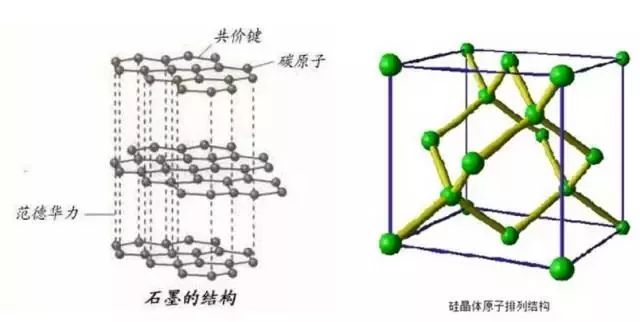
Figure 2. Structural comparison between graphite and silicon
Despite its advantages, silicon has a critical drawback as a lithium battery anode. During charging, lithium ions intercalate into the silicon lattice, causing a volume expansion of up to 300%, which leads to structural degradation and mechanical stress. This volume change can cause the anode to crack or delaminate from the current collector, leading to electrochemical corrosion and reduced battery safety and lifespan. Furthermore, repeated expansion and contraction disrupt the solid-electrolyte interphase (SEI), increasing lithium ion consumption and decreasing battery capacity over time.
To address these challenges, researchers have developed silicon-carbon composite materials that combine the benefits of both components. These composites are typically classified into two categories: conventional silicon-carbon composites (e.g., silicon-graphite, silicon-MCMB, silicon-carbon black) and advanced silicon-carbon composites (e.g., silicon-carbon nanotubes, silicon-graphene). Different combinations lead to various structures and methods of fabrication.
One common structure is the "walnut" structure, where porous silicon is filled with carbon to create a stable framework that accommodates volume changes during cycling. This design improves electrochemical performance, maintaining a reversible capacity of 1459 mAh/g at 1 A/g after 200 cycles. Another popular approach is the core-shell structure, where carbon coats the surface of silicon particles, preventing aggregation and reducing volume expansion. Some researchers have even developed a double-layer coating system to further enhance structural stability.
The ternary embedded structure involves integrating silicon with carbon nanotubes or graphene, creating a network that enhances conductivity and absorbs expansion. Finally, the "watermelon" structure, developed by the Chinese Academy of Sciences, features a layered design that effectively reduces particle fragmentation and improves cycle life.
The preparation methods for silicon-carbon composites include ball milling, chemical vapor deposition, and sputtering, among others. These techniques allow for a wide range of structures tailored to optimize battery performance.
In terms of market development, companies like Shanshan, Jiangxi Zijing, and BYD have already started producing silicon-carbon anode materials. Tesla, for instance, uses a 10% silicon-based composite in its Model 3, achieving a capacity of over 550 mAh/g and an energy density of 300 Wh/kg. In Japan, GS Yuasa and Hitachi Maxell have also introduced silicon-based anode technologies. With growing demand and technological advancements, silicon-carbon anode materials are expected to play a major role in the future of lithium batteries.
Core Alignment Fusion Splicer,Digital Core Alignment Fusion Splicer,Manual Fusion Splicer,Precision Alignment Fusion Splicer
Guangdong Tumtec Communication Technology Co., Ltd , https://www.gdtumtec.com